As per the latest Chinese, American and European Pharmacopoeia standards
Design required to comply with cGMP and GAMP specifications,,
Compliance with GMP, FDA certification requirements
| Product water quality | in accordance with Chinese Pharmacopoeia 2015 |
| Product process | double reverse osmosis (RO+RO) |
| Sterilization method | pasteurization |
| Control mode | Fully automatic control |
As per the latest Chinese, American and European Pharmacopoeia standards
Design required to comply with cGMP and GAMP specifications,,
Compliance with GMP, FDA certification requirements
3D analog manufacturing installation,
Customers participate in the whole process, and the detailed effects are displayed in advance, so as to guarantee the customer's needs in all aspects。
Modular design: more compact structure, simpler operation and maintenance。
Comprehensively understand the customer's requirements for product specification, product process, system configuration, etc. Customized design
Process flow of purified water equipment

Optional disinfection methods: activated carbon pasteurization, CIP cleaning system, ozone sterilization of distribution system, pasteurization of distribution system and pure steam sterilization of distribution system.
Remarks: Sendary Water system is customized according to user requirements (URS)

Select high-quality raw materials, and use professional instruments and equipment for strict quality inspection;
Modular 3D design, construction according to drawings;;
Welding is preferred for pipe connection, followed by clamp connection to control the growth of microorganism;
Automatic rail welding machine shall be adopted, 20% endoscope inspection shall be carried out after welding, and 100% endoscope inspection shall be carried out manually;
Carry out installation specification training before site construction。

On-line monitoring of produced water quality to meet customer requirements;
The design service life of the system is 15 years of safe and stable operation;
Provide GMP consulting service and GMP verification system documents;
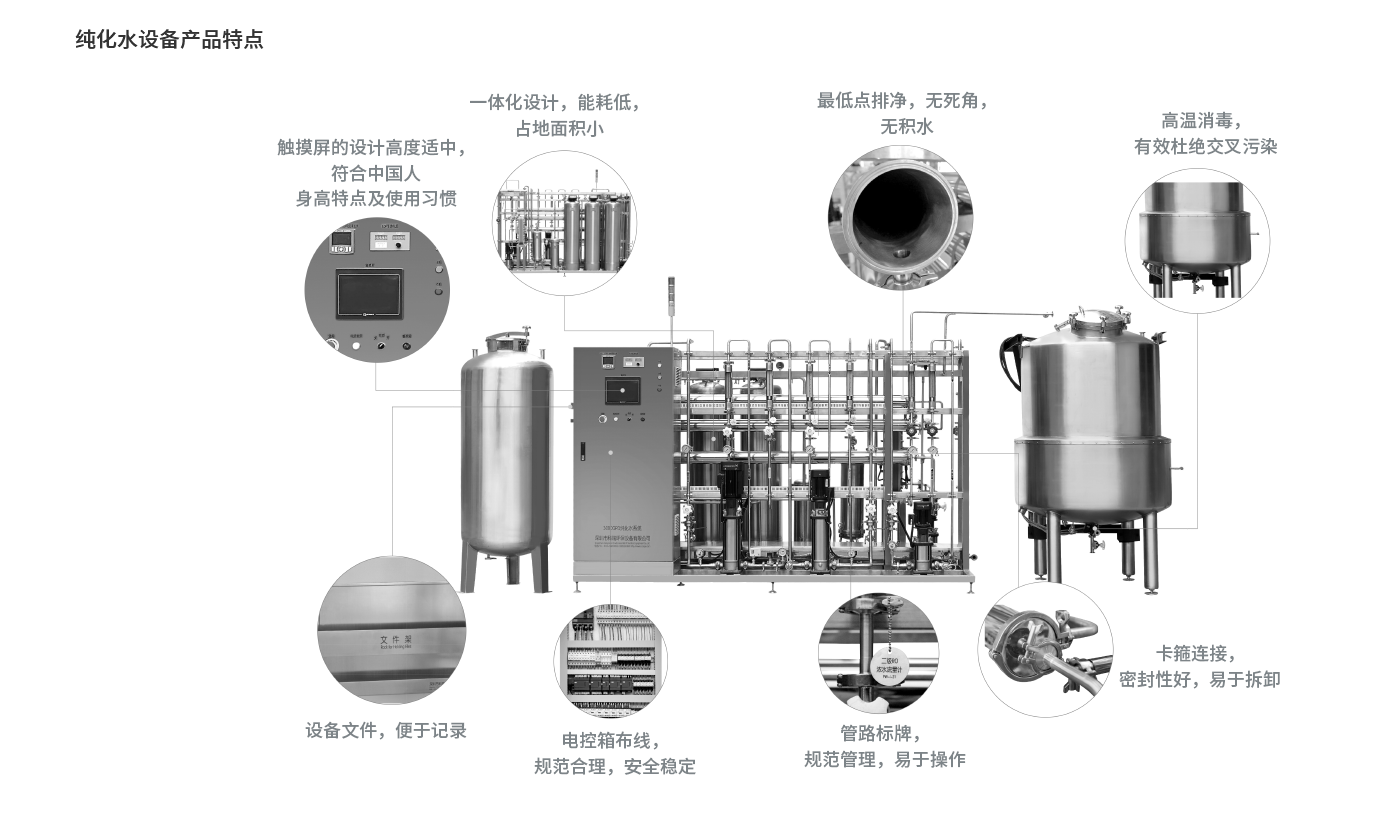
The system minimizes the risk of microbial growth, and the dead angle is less than 3D requirements;
Air blocking mode is adopted for drainage to avoid reverse suction and cross pollution. Drainage at the lowest point and 5 ‰ slope are set;
The return water flow rate of the circulating pipe is greater than 1m/s to control the growth of microorganism;;
The secondary concentrated water, EDI concentrated water and unqualified purified water are returned to the raw water tank for reasonable recycling, so as to save the water consumption of raw water and reduce the later operation cost.

具有三级管理权限,依次为操作员、管理员、高级管理员。每个登录帐号,有相应的登录密码(密码可修改),系统5分钟后自动注销,防止未授权人员进入系统误操作,参数可恢复出厂设置;
在系统关键的地方设置过载、高压、低压保护,所有故障、警报系统自动记录;
控制线路采用24V安全电压,确保操作人员和设备的安全。
多方内部通讯系统,在任意层的工作站点,均能相互浏览运行状态;
系统发生故障将短信通知对方,并能实现手机物联,观察运行状态。

融入人机工程学设计,触摸屏、开关、仪器、仪表、取样等位置的高度,符合国人平均身高操作;
一键式启动,操作、维护简单方便;
特殊设备具有操作平台及安全护栏。
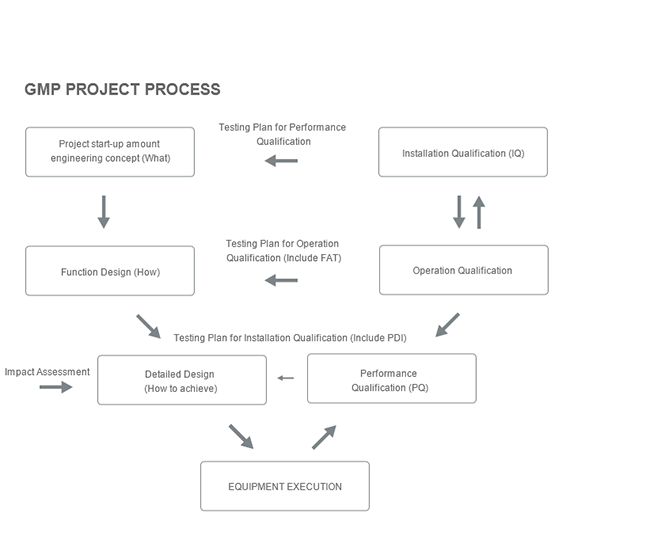
Provide full-process services including system design consulting, system equipment, GMP verification consulting, upgrading and renovation, operation and maintenance, etc.
Provide GMP, FDA verification consulting and system DQ, FAT, IQ, QQ, SAT, PQ verification data
Pre-sale: URS demand analysis
During sale: design, installation, debugging, verification, training
After-sales: rapid response, long-term service
Comprehensive understanding of customer needs for product specifications, product processes, system configurations, etc. Tailored design

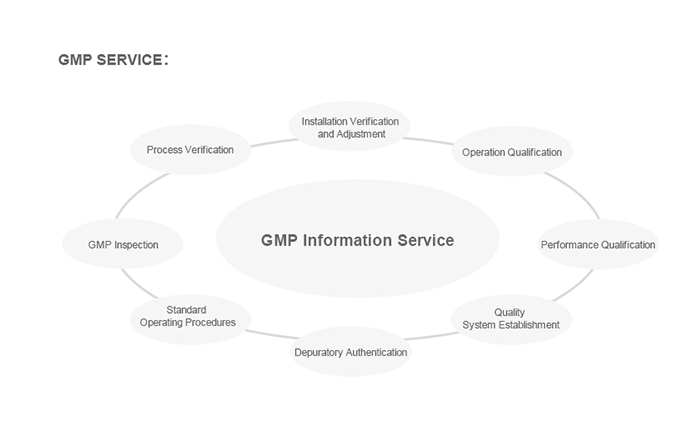
![]() Provide complete GMP system validation documents.Professional validation services throughout the project;
Provide complete GMP system validation documents.Professional validation services throughout the project;
>Free installation provides users with all the necessary conditions and work support for FAT testing;
>Provide users with free DQ, FAT, SAT, Q, OQ, PQ verification documents required for equipment;;
![]() International standards, in line with the requirements of the Chinese Pharmacopoeia, the European Pharmacopoeia, the British Pharmacopoeia, FDA, etc.;;
International standards, in line with the requirements of the Chinese Pharmacopoeia, the European Pharmacopoeia, the British Pharmacopoeia, FDA, etc.;;
![]() Striving for rich experience can avoid unnecessary design defects for you;
Striving for rich experience can avoid unnecessary design defects for you;
![]() High efficiency work can shorten the project cycle for you;
High efficiency work can shorten the project cycle for you;
>Provide users with guidance and related technical services during the installation of equipment for free;
>Provide free on-site equipment debugging for users to ensure that the equipment quality and related technologies meet contract requirements until the user completes SAT well acceptance and delivery for use;
>Free technical operator training for users until they are familiar with equipment and process technology and have the ability to independently operate, maintain, and repair the equipment (establish and provide SOP documents for customers)。


1. Train 1-2 equipment operation and management personnel to participate in the entire process of on-site installation to ensure that operators fully understand the equipment process principles.
2. Provide systematic technical information documents and comprehensive training, guide the completion of standardized operation procedures (SOP), establish operational inspection processes and systems.
3. After training, both practical and theoretical examinations will be conducted. A score of 90 in theory and 100 in practical operation is considered as passing the examination. Only those who pass the examination can take up their posts.
4. Provide updates and upgrades of equipment technical data and training materials as needed.
5. According to the operation and management personnel's use of equipment, they can receive targeted refresher training at Kerui Company.


1. After receiving the notification of equipment failure maintenance, propose a solution within 30 minutes and provide on-site service within 72 hours.
2. The equipment design, manufacturing, verification, and operation data are carefully preserved for 15 years, and a customer-specific file is established for easy access at any time, enabling traceability for equipment managers.
3. Set up a customer file management center,where after sales professionals can update。
4. After-sales professionals proactively conduct telephone follow-ups, record relevant data, and professional engineers review the data.If there are any issues, they will communicate and resolve them in a timely manner, and provide methods to reduce operating costs, reminders on maintenance and consumables replacement, and other related matters at any time.
5. The equipment is provided with vulnerable spare parts, and the company's warehouse is well stocked with commonly used consumables and accessories.Maintenance parts and materials are provided to the site for problem handling in the first time, and commonly used consumables and accessories are delivered to the site within 5-7 days.


Designed according to the latest Chinese, American, and
European pharmacopoeia water quality standards
Complies with ASTMD1193-2011 water quality standards
ISO13485 Medical Device Quality System Certification
Complies with GMP and FDA certification requirements
The water quality is better than the new Chinese Pharmacopoeia,
American Pharmacopoeia, and European Pharmacopoeia
Complies with GMP, FDA, and EU certification requirements
Compliance with Good Manufacturing Practice and Appendix
Complies with drug GMP guidelines, WHO World Health Organization
The water quality is better than GB17324-2003 bottled (barrel)
Hygienic standards for drinking purified water,
Conforming to Good Manufacturing Practice for Drugs,
Meet the requirements of QS and MSDS specifications,








